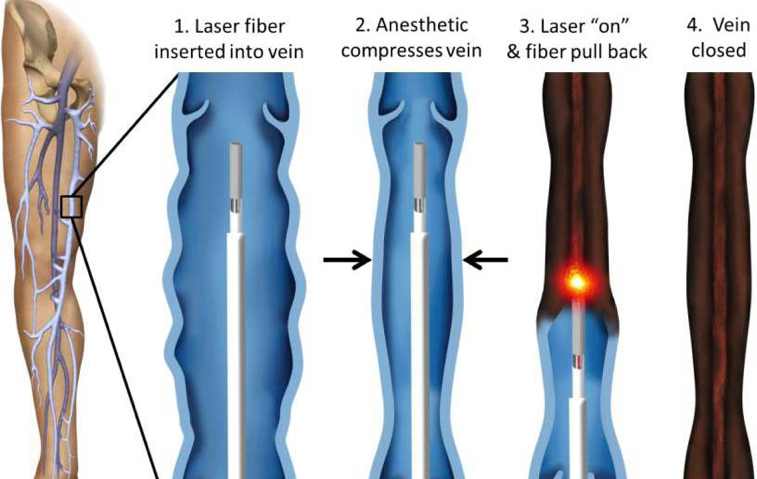
As for Root, he’s done with the medical business.
A year after his acquittal, Root quit VSI, and the company was sold for $1 billion.
He also left the medical device industry because he is unwilling to take the risks associated with a business that could make him the target of a malicious gov- ernment prosecution at any time, particularly under the Park Doctrine.
But he’s not done with federal prosecutors.
Root wrote a book about his experience, Cardiac Arrest: Five Heart-Stopping Years as a CEO on the Feds’ Hit-List. He also continues to give lectures about the tactics used by federal prosecutors in their quest to get a conviction and shut down any constitutionally protected talk about off-label uses of medical products.
Today, he’s involved in a small company developing electric-powered pontoon boats.
The government is not done with Root either.
In May 2017, Root was asked to participate in a panel discussion about his case at an American Bar Association summit on healthcare fraud. Prosecutors from the San Antonio U.S. Attorney’s Office who brought the charges were also supposed to be on the panel.
The Department of Justice not only refused to participate in any discussion with Root or about his case, it warned the ABA that if Root appeared, the agency would bar its lawyers from ever participating in the future.
Root’s invitation was rescinded.
The DOJ employed a similar tactic in 2018, when Root was invited to speak at a conference on the False Claims Act and qui tam enforcement actions. If Root participated in any way, DOJ officials warned, none of its attorneys would participate or attend.
Again, Root was disinvited.
Sen. Chuck Grassley, R-Iowa, at the time chairman of the Senate Judiciary Committee, sent two letters to DOJ regarding Root’s case. The first, sent in May 2016, sought an internal investigation of the threats and other hardball tactics used by prosecutors to secure the testimony of company employees.
In its response to Grassley, DOJ said the issues of misconduct had been raised by the defense in pretrial motions but had been rejected by the judge. It also said the agency’s Office of Professional Responsibility was looking into the allegations.
The second Grassley letter, dated March 2018, asked for the status of any internal investigation and an explanation as to why the DOJ apparently retaliated against Root by spiking his appearance at the two conferences.
In September 2018, DOJ replied that the internal investigation “found no evidence that Department attorneys engaged in professional misconduct” and closed the matter.
As to the conferences, the letter from Assistant Attorney General Stephen Boyd said DOJ lawyers get many invitations to speak, and they decide which to accept based on a variety of factors, including their workloads, the content of the conferences, and whether participation would be in the DOJ’s interests.
Root characterized the department’s refusals to participate in conferences as petty and an ironic attempt to further stifle his free-speech rights.
But that’s not the greatest damage that was done in his case, he said.
Nor is the personal peril he and VSI faced as a result of what he deems a malicious prosecution, or the $25 million that it cost to defend himself and his company.
The real damage is the lives lost because the innovative medical devices he or others at VSI might have developed were never invented, he said.
“Over the five years Vascular Solutions fought the government, we grew slower, invested less in R&D (research and development), hired fewer employees, and were robbed of $25 million we would have otherwise spent on productive medical activities,” Root said in his book.
“The interventional catheter I didn’t invent because I was sitting in a San Antonio courtroom will never be seen by anyone, and when you need it, you won’t even know it’s not there. Multiply that by all the medical device and pharmaceutical companies that have come under criminal investigation, and imagine how many medical breakthroughs you’ll never see.”
Download a PDF of this report